Can you spot the problems with these A level Biology exam question answers? These bloopers come up a lot - check you’re not going to make the same mistakes.
Read moreHow to Approach A level Biology Graph and Table Questions: Tips and Exam Question Pack
Get top marks when analysing figures, tables and images by avoiding common mistakes that students make
This article contains key vocabulary, a strategy for how to approach questions for success, a multichoice quiz with answers, and a big pack of past paper exam questions
Don’t panic, it’s only a graph
The single best exam tip for graphs and tables exam questions is to start by looking at the graph or chart itself. DO NOT LOOK AT THE QUESTIONS FIRST! This single thing will help you avoid the most common mistakes that students make.
But you also need to know what you’re doing. Which means you’ll need to be confident with these terms:
Background Knowledge / Vocabulary:
Independent Variable: The variable that you purposefully set to different values during the experiment
Dependent Variable: The variable that you measure during the experiment, which is unknown until it is measured
Replicate: Experimental data is often replicated - the same data point is recorded multiple times for the same conditions
Accuracy / Precision: Accuracy is how close the replicated values are to the correct value, and precision is how close they are to each other. If there is an unknown problem with the experiment, results can be very precise but have very low accuracy.
Range / Standard Deviation: The amount of variation in the data. A large range or standard deviation means that the replicated data had a broad range of results. A small range or standard deviation means they were much more similar in value. Range / Standard deviation is therefore a measure of precision.
Trend: What is the general relationship between the dependent and independent variables? When the experimenter increased the independent variable, what happened to the dependent variable? What shape is the graph?
How to Approach the Question:
1. Look at the graph or chart first!
Too many students look at the question first, get confused or panicky about what it is asking, and form preconceptions about what data they need. This then means they are then unable to look at the data clearly, and miss the information they actually need. Looking at the graph or chart first both makes the data easier to understand, and makes it easier to work out what the question is asking.
Trust me, this is a major factor in student success. If you only take away one thing from this article, always look at the graph or chart first.
2. Don’t panic if it’s about something totally unfamiliar
Students can get very thrown if the question is about an organism or molecule that they have never heard of before (the exam boards do this a lot). This sudden panic makes it hard to think clearly.
Remember - if you have covered all the course material, even if the question is about something weird and new then all the information you need will be in the data. The things that look scary are just surface details. If the question was “Fred gave James two apples, how many apples does James have” you wouldn’t need to know who these people were to answer the question.
But don’t just dive in to the details of the data …
What’s going on here?
3. Understand the format
Don’t waste time looking at the actual dots or numbers until you understand how the data has been presented. Check every aspect methodically. It’s too easy to make assumptions based on previous graph/table formats you have seen - this one might be different!
Look at the headings / axis labels and units. What is the data showing?
Identify the independent variable and the dependent variable. If possible, it’s helpful to label them “IV” and “DV”.
What type of data is shown? Is it averages? Does it include a Range or Standard Deviation?
Graphs: Check the axis labels. Have they plotted rate or time, mass/volume or concentration? Often students assume enzyme graphs have rate on the y axis - but sometimes they don’t!
Tables: Check: is the Independent Variable in the first column? Is the data in each row consistent?
4. Look at the data
Now you understand its context, look at the actual dots or lines or numbers. Check:
Does the Range overwhelm differences in values: Do the range bars or standard deviation bars overlap? If they do, then there is significant overlap between the populations of replicated results that were used to calculated the average values.
Unspecified Ranges: If there are replicates but no range bars or standard deviation has been calculated, how broad does the range look when you compare the replicated data values to their mean?
Trends: What trends can you observe? Then think about what principle of biology is being shown by the the trends.
Now think about what it all actually means:
Values: How would you explain the highest value, the lowest value, the point at which the line crosses the x axis,
Range: How would you explain the largest range? How would you change the method to reduce the spread in the data?
5. Ok - NOW look at the actual questions
Try to see past the detail. How does this data/question relate to things you have studied?
Your working memory can easily get overloaded with details, making it hard to think. If the examiners have introduced a new organism, its name won’t be important. What might be important is the environment in which it lives, or its interactions with other organisms. You know what data you have, and what the questions are, so pick out what actually matters here. Is this a question about enyme reaction rates? Or about surface area to volume ratio?
This is why it’s useful to look at the data first - you will be able to look at it with a clear eye, making it easier to pick out how it’s relevant to the material you have studied.
6. Give the required information
Avoid the common mistakes that lose students marks:
If they say you should use the data, you must either quote it, or show how you have used in in a calculation
Refer to the axis/data labels wherever possible. Don’t say “the graph goes up”, do say “the saturation of haemoglobin increases”
A-Level Biology Past Paper Graphs and Charts Exam Questions:
Got all that? Ok! Here are some questions for you to practice.
And remember - don’t read the questions until after you have made sense of the graph or chart.
If this post has been helpful, please like ❤️ below and share with your friends.
This article was written by Dr Jenny Shipway with guidance and editing from Tom. Tom has over 26 years experience specialising in A level Biology teaching and tuition, and has helped many students achieve top grades in the subject.
Transport in Animals: Haemoglobin, Oxygen Dissociation Curves, and the Bohr Effect
Haemoglobin, Oxygen Dissociation Curves, and the Bohr Effect
Red blood cells stuffed full of haemoglobin. Their bright red colour tells us their haemoglobin is in the form of oxyhaemoglobin, with bound oxygen.
This article includes an explanation of the topic followed by a short multiple-choice quiz with answers, and a collection of past-paper A-level exam questions for you to try.
Single-celled organisms absorb oxygen directly from their surroundings for use in aerobic respiration. But animals have cells buried deep within their bodies, far from the outside world. For this reason, the ability to transport essential substances like oxygen, and to remove waste products like carbon dioxide, is crucial for all animals.
This A-level Biology topic is challenging, but it’s really important to build an understanding of the mechanisms behind these processes. You’ll need to understand exchange and transport, and get to grips with the role of haemoglobin in transporting oxygen and carbon dioxide. And you really will need to understand it properly - as with most A-level topics, memorisation is not enough.
The really clever bit about oxygen transport in animals is the way that cooperative binding affects haemoglobin’s oxygen dissociation curve. Master that and you’ll be well on your way to getting full marks in this topic.
Vocabulary (for reference, don’t worry if you don’t know all these yet!):
Partial Pressure: The pressure exerted by one particular gas in a mixture.
Haemoglobin: A protein found in red blood cells. Able to bind oxygen, carbon dioxide, and other ligands.
Oxyhaemoglobin: Haemoglobin bound to oxygen, as occurs during oxygen transport.
Carbaminohemoglobin: Haemoglobin bound to carbon dioxide, as occurs during carbon dioxide transport. Yes it has a funny name! Be careful not to confuse it with carboxyhaemoglobin, which is hemoglobin bound with carbon monoxide.
Haemoglobinic Acid: Haemoglobin bound to a proton, as occurs during carbon dioxide transport.
Cooperative Binding: The phenomenon where the first oxygen molecule to bind to one of haemoglobin’s binding sites increases the affinity of the remaining binding sites for oxygen. And the first to release makes it easier for the others to release.
Carbonic Anhydrase: An enzyme that catalyses the reversible conversion of carbon dioxide and water into carbonic acid.
HCO3–: The bicarbonate ion, one of the ways in which carbon dioxide is transported in the blood.
Chloride Shift: The movement of chloride ions into red blood cells as bicarbonate ions move out, maintaining electrical neutrality during the transport of carbon dioxide.
Oxygen Dissociation Curve: A curve on a graph that shows how saturated with oxygen haemoglobin is at different partial pressures of oxygen. It curves because of the effect of cooperative binding. The position of the curve is affected by pH.
Partial Pressure:
None of this will make any sense if you don’t understand what partial pressure is, so that’s a good place to start. Partial pressure is the pressure exerted by one particular gas in a mixture. You can increase the partial pressure of oxygen molecules (O2) in air by either having greater overall air pressure, or by a larger proportion of the air being oxygen.
The percentage of Oxygen in the air is the same on Everest as at sea level.
When you ascend Everest, the percentage of oxygen in the air does not fall, but the air pressure does. And so the partial pressure of oxygen in your alveoli falls.
When you re-breathe the same air, the air pressure does not fall, but the percentage of oxygen in the air does. And so the partial pressure of oxygen in your alveoli falls.
Gases still have partial pressures when they are in solution. The partial pressure of carbon dioxide is really high in an unopened can of coke. And the partial pressure of oxygen in your body fluids must be carefully regulated if your cells are to survive.
Remember that oxygen is the final electron acceptor in oxidative phosphorylation on the inner mitochondrial membrane, where it becomes water (H2O). Respiration therefore removes oxygen molecules (O2) from the body, lowering its partial pressure. The harder a tissue works, the more ATP will be produced from aerobic respiration, and the more oxygen atoms will be moved from O2 into H2O.
Respiration therefore lowers the partial pressure of oxygen (O2) in the tissues. Especially in energy-hungry tissues like muscle.
The partial pressure of carbon dioxide (CO2) also changes in the body. Respiration produces carbon dioxide by decarboxylation of pyruvate in the link reaction and citrate (etc) in Krebs cycle. As the partial pressure of oxygen falls, that of carbon dioxide increases. It’s not the same oxygen atom (remember the one from O2 ended up in H20), but the two processes are closely connected.
Respiration therefore increases the partial pressure of carbon dioxide in the tissues. Especially in energy-hungry tissues like muscle.
This is why animals need a system to support gas transport - it is necessary to move oxygen in to the tissues from the outside world, and to move carbon dioxide out of the tissues to excrete it out of the body.
Haemoglobin’s role:
Haemoglobin plays a pivotal role in transporting oxygen and carbon dioxide in the bloodstream. Haemoglobin is a protein containing four polypeptide chains, each of which provides a binding site that can bind reversibly with oxygen or protons (and some other things too). The timing of its binding and release of its ligands depends on various factors:
Haemoglobin - there are four polypeptide chains, here two are shown in green, and two in red (apologies to anyone who is colour-blind, I didn’t make this graphic). Each polypeptide chain is folded to create a subunit of the protein, and each subunit provides a binding site which can bind one Oxygen molecule.
Oxygen Binding: When oxygen levels are high, haemoglobin binds with oxygen molecules, forming oxyhaemoglobin. This happens in the lungs, where blood is brought close to the surface of the alveoli. Oxyhaemoglobin is bright red; this is what gives blood its red colour.
Cooperative Binding: When the first oxygen molecule binds, haemoglobin changes shape in a way that increases the affinity of its remaining binding sites for oxygen. This results in a rapid increase in oxygen saturation once the first oxygen molecule binds to haemoglobin. There is a similar effect on release of oxygen - releasing one oxygen makes it easier for the others to be released.
Carbon Dioxide Binding: Haemoglobin binds a small percentage of the carbon dioxide produced by the body tissues to help transport it back to the lungs. Haemoglobin bound to carbon dioxide is called carbaminohemoglobin, and is a dark maroon colour. It is not blue!
Carbon Dioxide transport:
About 30% of the carbon dioxide produced by body tissues gets directly bound by haemoglobin for transport. A lot more - about 70% - travels in the blood plasma. This is possible because of the action of the enzyme Carbonic Anhydrase.
Carbonic Anhydrase: Inside the red blood cell, the enzyme carbonic anhydrase catalyses the reversible conversion of carbon dioxide and water into carbonic acid. This acid then dissociates into bicarbonate ions (HCO3-) and protons (H+).
Haemoglobinic Acid: The positively-charged protons from the dissociated carbonic acid bind to the haemoglobin to form haemoglobinic acid. This keeps these positive charges inside the red blood cell.
Chloride Shift: In contrast, the negatively-charged bicarbonate ions are free to diffuse out of the red blood cell into the blood plasma. To maintain electrical neutrality, chloride ions (Cl-) diffuse into the red blood cell. This is known as the chloride shift.
Cooperative Binding and the Oxygen Dissociation Curve:
The much-feared oxygen dissociation curve. The really interesting thing is that it is not a straight line. You need to know why not, and why this is crucial for oxygen delivery to the body tissues.
The Oxygen Dissociation Curve is a graph plotting the partial pressure of oxygen (how much oxygen there is in the environment) against how saturated the haemoglobin is with oxygen.
The graph is always drawn with the partial pressure of oxygen on the X-axis, showing low oxygen to the left, and high oxygen to the right. Oxygen binding is plotted against the Y-axis, with the curve plotted higher at partial pressures where the haemoglobin is more saturated with oxygen.
Take a little while to work out exactly what the graph is showing, as it can be a bit confusing at first.
As you might expect, when there is more oxygen around, more oxygen gets bound. But it’s not quite as simple as that.
The sigmoid shape of the oxygen dissociation curve shows why haemoglobin is so ideally suited to its role in oxygen transport. The shape of the curve is explained by haemoglobin’s remarkable property of cooperative binding.
Haemoglobin has four tertiary domains, each with its own binding site. So it can carry four oxygen molecules. The really clever bit is that when the first oxygen molecule binds, this causes a change of shape in the protein that increases the affinity of the other three binding sites for oxygen. This increased affinity increases their chances of binding oxygen too. This all has the effect that once just one oxygen has bound, the haemoglobin’s binding sites are quickly saturated. And the opposite happens when oxygen dissociates - the dissociation of the first oxygen reduces the affinity of the other binding sites.
The way in which one binding event encourages others is called Cooperative Binding. This ‘all or nothing’ tendency affects the shape of haemoglobin’s oxygen dissociation curve by squashing it down at the bottom and up at the top, creating its famous sigmoid shape.
The sigmoid shape of the oxygen dissociation curve is crucial for efficient oxygen delivery to tissues. At low oxygen concentrations (e.g., in tissues with high metabolic activity), haemoglobin exhibits low affinity for oxygen, allowing it to release oxygen to respiring cells. Conversely, at high oxygen concentrations (e.g., in the lungs), haemoglobin exhibits high affinity for oxygen, facilitating its uptake from the lungs.
The Bohr Effect: Carbon Dioxide and the Oxygen Dissociation Curve
Notice that the right-shifted, red curve is lower than the blue one. This tells us that at high CO2, haemoglobin has LESS affinity for oxygen. (The colours don’t mean anything.)
Haemoglobin’s oxygen dissociation curve isn’t fixed in place. It can move to different positions depending on the pH.
The pH in the red blood cell is affected by the partial pressure of carbon dioxide (remember how it behaves during transport?). High levels of carbon dioxide indicate that the body is active and needs more oxygen.
Active muscles also produce lactic acid through anaerobic respiration, which further reduces the pH.
A low (acidic) pH has the effect of moving the oxygen dissociation curve to the right. This means that any any given partial pressure of oxygen, this high-CO2, right-shifted curve is lower than before.
It’s not enormously intuitive, but you can see this clearly if you draw a line vertically up through the graph at a chosen partial pressure of oxygen. Look at where it meets each curve. It will hit the right-shifted curve before it hits the original curve, because the right-shifted, high-CO2 curve will have lower oxygen saturation at this (or any) patial pressure.
That means that at any partial pressure of oxygen, the high-CO2 haemoglobin has a lower affinity for oxygen than before, and is more likely to release its oxygen into the tissues.
Reducing haemoglobin’s affinity for oxygen at high partial pressures of carbon dioxide helps it release oxygen in the active tissues that need it most.
Fetal Haemoglobin and Myoglobin
Haemoglobin sometimes needs to pass its oxygen to other, similar oxygen-binding molecules. It needs to pass it to myoglobin for oxygen storage in muscles, and to fetal haemoglobin to pass oxygen to the growing foetus.
These similar-but-different molecules have their own oxygen dissociation curves. If oxygen is to be passed to them, they must have a higher affinity for oxygen than normal haemoglobin. And this is what is seen. When you look at their curves, they are left-shifted with respect to haemoglobin. Draw a line up from any chosen partial pressure of oxygen, and it will hit the curve for normal haemoglobin first, because the fetal haemoglobin or myoglobin will have higher oxygen saturation.
This means that at any partial pressure of oxygen, they have a higher affinity for oxygen, and are able to bind oxygen that has been released by the normal haemoglobin.
Multiple Choice Questions (answers below):
What is the primary function of haemoglobin in the bloodstream?
a) Transporting nutrients
b) Transporting oxygen
c) Transporting waste products
d) Transporting hormonesWhich enzyme catalyzes the reversible conversion of carbon dioxide and water into carbonic acid?
a) Carbon dioxide synthase
b) Carbonic anhydrase
c) Haemoglobinase
d) Bicarbonate dehydrogenaseWhich acid is formed when haemoglobin binds with a proton?
a) Carbonic acid
b) Haemoglobinic acid
c) Hydrochloric acid
d) Sulfuric acidWhich ions move into red blood cells when bicarbonate ions diffuse out?
a) Sodium
b) Potassium
c) Chloride
d) HydrogenWhat effect does an increase in carbon dioxide concentration have on the oxygen dissociation curve?
a) It shifts the curve to the left
b) It shifts the curve to the right
c) It has no effect on the curve
d) It decreases the steepness of the curve
Multiple Choice: answers
1. Answer: b) Transporting oxygen
2. Answer: b) Carbonic anhydrase
3. Answer: b) Haemoglobinic acid
4. Answer: c) Chloride
5. Answer: b) It shifts the curve to the right
How did you do?
These questions were just a quick test to see if you can remember some of the key points. If you struggled a bit then go back and review the content. But also check that you really understand what’s going on with this topic. The exam board will intentionally phrase the questions to make it as difficult as possible for anyone to answer by having just memorised key facts.
When you’re ready, try some real exam questions:
If this post has been helpful, please like ❤️ below and share with your friends.
This article was written by Dr Jenny Shipway in collaboration with Tom
How to Revise A Level Biology: Learn the Language
A guest blog from Dr Jenny Shipway, who studied biochemistry at university and now works in science communication and education training.
The Language of Science
Words, Words, Words
One of the reasons I love biology is the wonderful language that comes with it. But learning so much new vocabulary can be a real challenge. And yes you’re going to need to learn it - both to understand the exam questions, and to communicate your answers clearly.
It helps - a lot - to use scientific language as much as possible from the very start of your studies. It might feel awkward, but fight the urge to slide into everyday speech for comfort, or to fudge the syllables of complex words. Consciously use scientific language so that it becomes a habit. And whenever possible, speak words out loud - the muscle memory will help you remember them. Using scientific language will require you to properly organise your thoughts, so being able to do this is also a great check that you really do understand a concept.
And it’s not just about remembering scientific words (although I have some tips for that below) - you will also need to know the words the examiners will use to describe what you have to do to get full marks.
Command Words
These are the words that will communicate what you need to do in exam questions. Fully understanding them will ensure you focus your efforts on the right things. However much accurate and interesting information you write down, if it’s not what the examiner was looking for then you won’t get the marks.
When you read an exam question, look out for words like these:
Evaluate: judge using available evidence
Show: provide structured evidence to reach a conclusion
Deduce: draw conclusions from the evidence provided
Find a list of useful command words here
Scientific Vocabulary
Communication is a core concept of science, and that communication has to be as clear as possible. There are a lot of scientific words that can help you achieve this clarity. But only if you use them correctly.
For example:
Accuracy / Precision: in academia, accuracy and precision are very different things. Accuracy is how close the values are to the correct value, and precision is how close they are to each other.
Repeatable / Reproducible: in science, “repeatable” means the experiment has been repeated by the same experimenter using the same equipment, and the same results were obtained. “Reproducable” means the same results are still obtained when the experiment is run by a different person, or using different equipment/techniques.
FInd a list of useful scientific vocabulary here
Jargon
Some molecules and processes have really complicated names. But they are not just random letters - they have coded meaning. When you see a new word, or need to remember one, look at it carefully and see how it breaks down. Most long biological words are constructed from coded fragments stuck together.
For example, “carbonic anhydrase” is “carbon” + “ic” + “an” + “hydr” + “ase”. What does this molecule do? Look below if you’re stuck.
Important prefixes and suffixes:
a- / an- : prefix meaning “not”. As seen in words like abiotic, anhydrase, and asexual. The “an” version is used when it goes in front of a vowel or h.
bio- : prefix meaning it’s about something living. As seen in words like biology, biochemical, biotechnology, biotic, and biomass.
cardi[o]- : prefix meaning it’s about the heart. As seen in cardiovascular, cardiopulmonary, cardiac.
cyto- : prefix meaning it’s about cells. As seen in cytoplasm, [endo/exo]cytosis, cytokinesis, cytokines.
endo- / exo- : prefixes meaning “inside / outside”. As seen in endoskeleton vs. exoskeleton; endotherm vs. exotherm; endocytosis vs. exocytosis; and endocrine vs. exocrine.
extra- : prefix meaning “outside / beyond”. As seen in extracellular, extraordinary.
glyco- : prefix meaning it’s something to do with glucose. As seen in glycolysis, glycosidic, glycogen, glycolipid and more.
hetero- / homo- : prefixes meaning “different / the same”. As seen in heterotrophic, homologous.
hydr : prefix relating to hydrogen or water. As seen in carbohydrate, hydrostatic, and carbonic anhydrase.
hyper- / hypo- : prefixes meaning “over / under”. As seen in hyperglycemia, hypothalamus and many more words.
-ase : suffix often use for enzyme names. As seen in amylase, polymerase, helicase, ligase, lactase and many more.
-in : suffix often used for protein names, no matter their function. As seen in actin, myosin, insulin, and opsonin. But keep your wits about you: not all “-in”s are proteins, for example penicillin is not.
-ic : suffix meaning “relating to”. As seen in abiotic, polymorphic, metabolic, antibiotic, genetic and many more.
-ose : suffix often used in the names of sugars. As seen in glucose, fructose and ribose. Complex carbohydrates sometimes use it - cellolose does, but starch and glycogen do not.
-some : suffix meaning “body” (ie a lump of stuff). These names are often given to things that have been spotted by use of a microscope. As seen in ribosome and chromosome. Also very often used for spheres of cell membrane: eg lysosome, acrosome and phagosome.
mono- : means one. As seen in monomer; monosaccheride, mononucleotide, monogenic,
di- : means two. As seen in dimer; dipeptide, dihydrogen oxide (water!), and many other words. But of course other words just happen to start “di-” and so you have to look at the rest of the word to be sure.
tri- : means three. As seen in trimer, adenosine triphosphate (ATP) and others.
[there are other ones for higher numbers, but they are used less often]
poly- : prefix meaning many. A polymer is something made of repeated units stuck together (one unit is a monomer, two are a dimer, etc). As seen in polypeptide, polysaccharide, and polynucleotide. Also in words like polymorphic.
There are huge numbers of these word fragments - this list just contains some of the most important for A level Biology. Try to spot them as you go along - this will make it easier to remember the names of new process and molecules by relating them to their function. And maybe consider building up a bank of flashcards to help get them really stuck in your memory. If you can master these, learning new scientific jargon will be a lot easier.
Most importantly, make sure you’re not skipping over the middle bits of these words! Can you spell them from start to end? This will be a lot easier if you think about their entire structure, rather than just the beginning and end. Remember you won’t get the mark if you mess up the middle.
This is one of the reasons that speaking these words out loud helps - your brain might lie to you that you remember the middle bit, but speaking it out loud (without looking at the spelling!) is a great check for this.
If this post has been helpful, please like ❤️ below and share with your friends.
Key Concept: Polar and Charged Molecules
The similarites and differences between non-polar, polar and charged molecules (or parts of molecules) are really important. You must understand the difference between polar and charged molecules if you are going to make sense of molecular structure, and of the ways in which molecules interact.
If you’re unsure if water is a polar molecule or are wondering whether ions are polar, this article is for you.
This molecule is polar, but not charged. All is explained below.
Getting this topic straight in your mind will make it much, much easier to grasp key concepts like why glucose dissolves in water, why other things don’t, and how neurons and mitrochondria use membranes to create ion gradients for their function. And you’ll need to understand hydrogen bonding of polar groups to understand how DNA and proteins adopt defined structures.
The fact the molecules are called ‘polar’ and ‘charged’ is part of the problem - this can be pretty confusing! So don’t rely on their names to understand what’s going on.
Let’s start from scratch:
How are Polar and Charged Molecules different from Non-Polar, Uncharged Molecules?
All molecules contain atoms. And all atoms contain positively-charged nuclei and negatively-charged electrons.
In a non-polar, non-charged molecule, these positive and negative charges all neatly cancel each other out. As far as other nearby molecules are concerned, a non-polar molecule behaves as though it has no charges at all.
In both polar and charged molecules, the molecule has regions of positive and/or negative charges that can affect nearby molecules (or even other parts of the same molecule - as happens in proteins and DNA).
What’s the difference between Polar and Charged Molecules?
Polar molecules are charged-balanced overall but have unevenly distributed electrons. This gives them a little bit of a charge in certain places.
Charged molecules do have an overall charge. They have at leat one full unit of charge on at least one atom. (A unit of charge being equal to the magnitude of one electron).
You will also hear about polar and charged groups, which are a part of a larger molecule, where that part (group of atoms) has these properties.
Now, you might read that and think yes! I’ve got it! But to really understand it - and more importantly to remember it - you are going to need to linger a while and spend a bit of time thinking about this. It’s worth going through it all carefully step by step - this will also check your understanding. Have a good think about where those electrons are. Too many students trip up on this topic.
So let’s look at what it means to be non-polar, polar or charged. And then how that affects the behaviour of these molecules.
First step: What’s the difference between Unpolar and Polar Molecules
Atom
The atoms that make up molecules each have a postively-charged nucleus and a cloud of negatively-charged electrons.
Different types of atoms have different numbers of charges.
This means that even non-charged, non-polar molecules contain charges! They just cancel each other out so you don’t notice them.
Non-polar
When you make a molecule out of atoms, electrons are shared between neighbouring atoms. The electrons become one big shared cloud. This makes a covalent bond.
The diagram shows a non-polar molecule with two atoms. In a non-polar molecule, all the charges are balanced, cancelling each other out.
Because the charges are distributed evenly, and cancel out overall, the molecule behaves as though there are no charges (in terms of its electrostatic interactions with other nearby molecules).
So why are they called “non-polar”? To understand that, you need to understand what polar means.
Polar
It turns out that some types of atomic nuclei just LOVE electrons. Like, they are particularly greedy for them. Oxygen, for example.
These greedy atoms yank the electron cloud over towards their nucleus, away from the nucleus of the other atom.
The other atom no longer has enough negative charge to cancel out its positively-charged nucleus. While the greedy one has more negatively charged electrons than it needs.
The charges no longer cancel each other out. The other atom now has just a little bit of a positive charge, and the greedy one has just a little bit of a negative charge.
This is a polar molecule. Its atoms still share one electron cloud, so they are still covalently bonded. But the small charge in charge distribution mean it will now interact differently with its environment.
Overall, the charges still cancel out. They are just unbalanced so that there are places with just a little bit of charge.
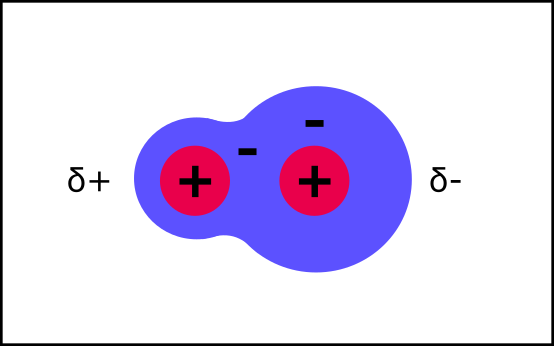
Saying "just a little bit” of charge is a pain, so instead the delta symbol is used to show this.
δ+ = just a little bit of positive charge
δ- = just a little bit of negative charge
This can also happen to just one part of a molecule. A good example is a hydroxyl group (OH). The oxygen pulls the electrons toward it, so that there is just a little bit (δ) of charge on the oxygen and hydrogen atoms.
Molecules with hydroxyl groups are polar. Look at glucose - it has loads of hydroxyl groups; this is what makes it a polar molecule. This is important for how it behaves in water, but before we get to that, let’s look at how charged atoms/molecules are different:
Second step: What’s the Difference between Polar and Charged Molecules?
Polar
A polar molecule has no overall charge. The charge of its positive nuclei exactly cancel out the charge of its negative electrons.
The charges are just unevenly distributed, giving a little bit (δ) of positive charge to one atom, and slight negative charge to another atom.
In biology, you’ll normally find it’s a hydrogen atom that has had its electrons yanked away and is now carrying a little bit of positive charge.
Charged
Now look at this. These atoms are not sharing a cloud of electrons - the big one has gone all-in and taken the whole lot for itself.
No shared electron cloud means there is no covalent bond.
No covalent bond means they are no longer a single molecule, but rather two separate atoms … well, except that they’re not even atoms any more …
The atoms no longer just have just a little bit (δ) of charge. The one on the left has lost an entire electron’s worth of charge. Losing negative charge means that overall it is now (properly, not just a little bit) positively charged.
The one on the right has a whole electron’s worth of negative charge more than it needs to cancel out its positive nuclues. It is now (properly) negatively charged.
Because they are (properly!) charged, we no longer call them atoms. Instead they are ions.
Water experiences this sort of electron-theft.
Sometimes it exists as the polar H20 molecule, but sometimes its oxygen gets even more greedy and the molecule dissociates into H+ (a hydrogen ion, aka proton), and OH- (a hydroxyl ion).
This dissociation, and the reforming of H20, is happening all the time in normal liquid water.
Note that the ions each have an overall charge, unlike the polar water molecule where the small charges cancel out.
Bigger Molecules
Atoms that are negatively charged due to having extra electrons, or that are positively charged because they lack electrons, often occur in large molecules too.
Where positive charges are found, it helps to think about this as a positively-charged H+ having been added to the molcule.
Here’s an amine group. It’s just part of a larger molecule, which goes off the edge of the image.
It can exist either as —NH2, or it can add on a proton (H+) to become —NH3+.
In living organisms, there are plenty of available protons (remember how water dissociates?). So amine groups like this usually exist as the charged version.
This is not a polar group, it is charged. (Ignore the shape of the electron cloud for this one, the important thing is that there is an overall charge of +1 because of that extra proton).
Electrostatic Interactions
So. Polar molecules are uncharged overall but have just a little bit (δ) of charge in various places. While charged molecules have a big whack of charge due to having lost an electron or having gained a proton. Why is this difference so important?
It’s to do with how polar and charged molecules interact with their environments. It’s not the same.
Hydrogen bonds
Some polar molecules, like DNA, proteins and water, can form hydrogen bonds between the atoms that have the unevenly distributed charges. These are a special type of weak bond.
Water LOVES making hydrogen bonds - this is why it can hold itself together into a droplet.
Notice in the picture that the water molecules remain separate and can still move around. It doesn’t take much to pull a single hydrogen bond apart. Which is why water can still be poured and stirred around with no trouble.
Water is a polar, hydrogen-bonding molecule, and this explains its properties as a solvent. Molecules like glucose can dissolve in water because they are similarly polar and able to make hydrogen bonds.
Hydrogen bonds are also really important in understanding DNA and protein structures.
These molecules hydrogen-bond to themselves. Each individual bond is weak, but multiple repeating bonds work together to hold the structure into shape.
Protein secondary structures are held together by hydrogen bonds.
The image here shows hydrogen bonds between Guanine (G) and Cytosine (C) in DNA. The hydrogen bonds are shown as dotted lines.
To get a feeling for the strength of hydrogen bonds, think about what happens if you spill water on a book, close it, and let it dry. You know how the pages stick together? This is because hydrogen bonds have formed between the pressed-together pages. When you peel them apart, you are pulling these hydrogen bonds apart.
Dissolving ions
Charged ions like Cl-, Na+ and K+ can’t form hydrogen bonds, but they can still dissolve in water because they can form favourable electrostatic interactions with the water molecules.
This diagram shows salt (NaCl) dissolved in water.
Hydrogen bonds are in yellow. And electrostatic interactions between the charged ions and the polar water molecules are shown in green.
See how the water molecules organise around the ions to provide the opposite charge to that presented by the ion.
Non-polar molecules like lipids cannot form electrostatic interactions with water molecules. And so for this reason, non-polar molecules do not dissolve in water. If you could somehow spread a bunch of non-polar molecule through a glass of water, this would cause all sorts of problems because the water molecules next to the non-polar molecules would be unable to satisfy their charges. Water prefers to hydrogen bond to itself, and it would do so, squeezing the the non-polar molecules out to cluster together in undissolved lumps.
Whisk up a teaspoon of oil in a glass of water and watch - you can see this happening. The oil ends up as a separate layer on the surface. Or get a small glass of oil and carefully put a drop of water on top; the water will ball itself up, hydrogen-bonding to itself and minimising the amount of contact it needs to make with the oil.
This is why membranes don’t dissolve in the cytoplasm. The water molecules would much rather hang out with other water molecules where they can make all those lovely hydrogen bonds. Non-polar molecules are called hydrophobic, or “water-hating”, but to be honest that’s a bit unfair because really it’s the water is excluding them, rather than the other way around.
This also means that non-polar molecules can’t act as solvents for polar molecules or charged ions. The reason being the same: they can’t offer any way to satisfy the polar/charged molecules’ hankering for favourable electrostatic interactions. This is why ions (Na+, K+, H+ etc) cannot dissolve into, and move through, membranes. Which is absolutely vital to understand if you want to make sense of how neurons, mitochondria, and chloroplasts function (and many other things in biology besides).
Ionic bonds
Charged molecules have ‘proper’ charges. They interact more strongly through electrostatic interactions to form ionic bonds.
Here is a positively charged amine group (NH3+) forming an ionic bond with a negatively charged hydroxyl group (OH-).
They are not sharing an electron cloud, so this is not a covalent bond.
Maybe these charged groups are both parts of the same protein (ie from different R groups). If so, this interaction may be important in defining the protein’s tertiary structure.
Or maybe it’s an interaction between an enzyme and its substrate?
Ionic bonds are really important for controlling what binds with what - and what doesn’t. Negatively charged groups will repel other negative charges. And positive will repel positive. This prevents incorrect structures forming.
In summary:
Polar molecules are charge-balanced overall but have unevenly distributed electrons. This gives them a little bit ( δ ) of a negative charge on one atom, and a little bit ( δ ) of positive charge on another. In biology, these weak charges often form hydrogen bonds, or favourable electrostatic interactions with ions.
Charged molecules have an overall charge. They have at leat one full unit of charge on at least one atom. (A unit of charge being equal to the magnitude of one electron). These stronger charges can form ionic bonds with each other.
This article was written by Dr Jenny Shipway
If this post has been helpful, please like ❤️ below and share with your friends.
Improve your Exam Answers -Small changes that will get you more marks - UPDATED JAN 2023
How to change the language you use in A level Biology Answers, and get better grades
Read moreXylem and Transpiration Question - How would you answer this one ?
Pre-U synoptic exam question - Xylem and transpiration. Transport of Water in Plants
Read moreMuscles, Calcium, mitosis and potency - Try this excellent synoptic question
Outstanding synoptic question on muscles and contraction. Excellent practise
- if you use then please like and share
Read moreRespiration and Enzymes/Protein structure ... Hard Synoptic A-level Biology Question
This is an outstanding synoptic question from a Pre-U Biology Paper. Covers Protein Synthesis, Enzymes, Heart Disease, Respiration ....
Read more